Innovation Hub
Health Challenges
Cancer is still one of the top causes of death, with lung cancer affecting more than 2.4 million people each year, breast cancer more than 2.3 million, and pancreatic cancer taking nearly 500,000 lives annually. Beyond cancer, autoimmune diseases like rheumatoid arthritis, multiple sclerosis, and inflammatory bowel disease (IBD) affect over 50 million people worldwide, often leading to lifelong disability and lower quality of life. Infectious diseases, from the flu to new viral outbreaks, continue to impact billions, with lower respiratory infections alone causing nearly 3 million deaths each year.
At SJP Biotec, we develop breakthrough treatments that tackle these urgent health challenges.

Cellular targets
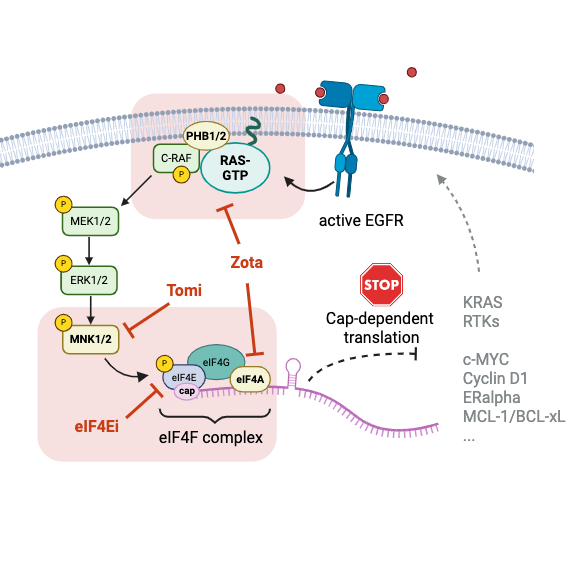
Our research is centered on uncovering drivers of cancer progression, autoimmune activation, and infectious disease - and how to stop them. We focus on two key cellular processes: the RAS signalling pathway, a major switch that helps cells grow and stay alive, and translation initiation, the step that activates the cellular protein factory to synthesize proteins. Cancer cells often hijack these two processes to fuel growth and metastasis.
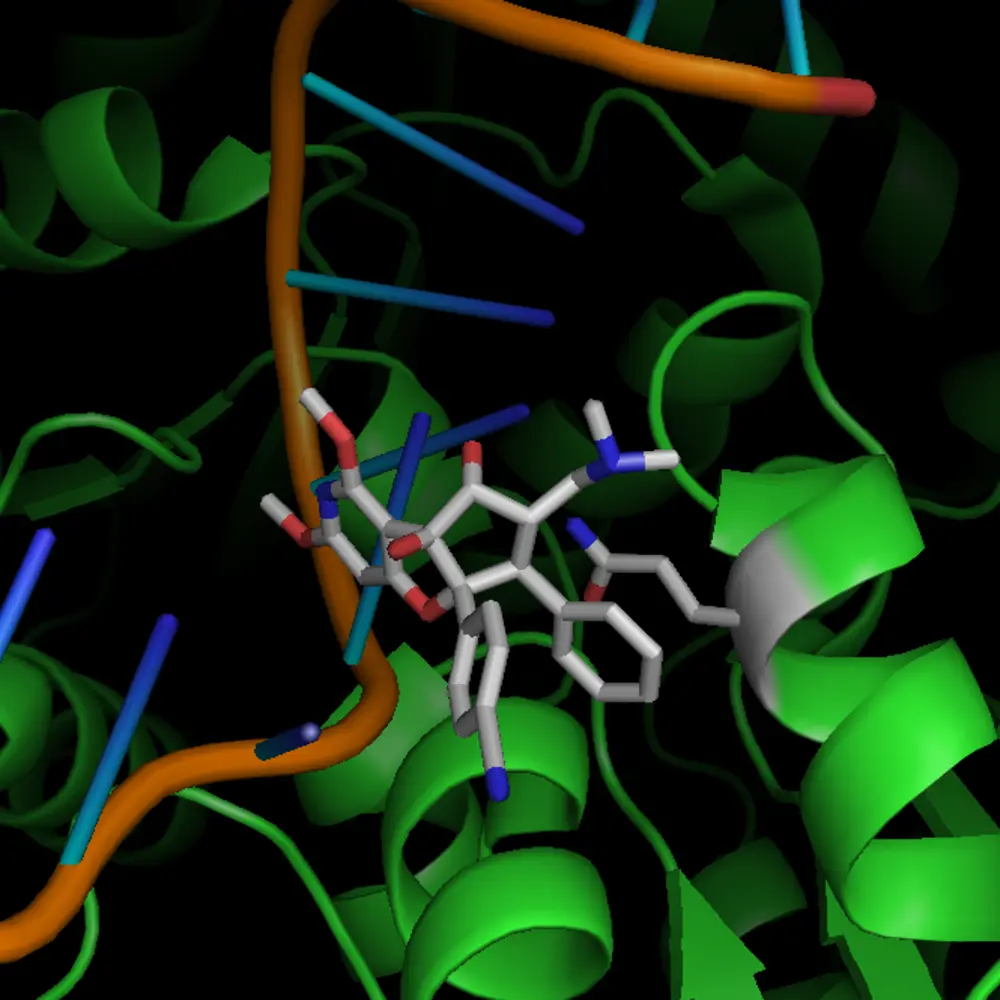
Drug Classes
Flavaglines are natural compounds derived from the Aglaia plant, which have demonstrated remarkable anti-cancer and anti-viral effects in preclinical studies. Having uncovered their mechanisms of action, they serve us as blueprints for the development of highly effective next-generation therapeutics designed to outsmart disease and enhance patient outcomes:
- Small Molecule Inhibitors: Designed to precisely block the activity of specific proteins, such as kinases. Their small size allows them to easily enter cells, making them ideal for targeting intracellular drivers of disease.
- Antibody-Drug Conjugates (ADCs): Often called "biological missiles", ADCs combine the precision of an antibody with a cytotoxic drug, minimizing damage to healthy tissues and reducing systemic side effects.
- Heterobifunctional Degraders: They work by directing the cell's own waste-disposal system to the target protein, offering a more durable and comprehensive therapeutic effect.
Clinical Trials
They represent the final and most crucial stages in the drug development process. Phase 1 trials primarily focus on assessing the safety, tolerability, and pharmacokinetics of a new drug. Conducted in a small group of healthy volunteers they usually take several months to complete. Following this, Phase 2 trials evaluate the drug’s efficacy and optimal dosing in a larger group of patients, while continuing to monitor safety. Phase 2 studies typically last from several months up to two years. Together, these phases provide essential data that determine whether a drug can proceed to Phase 3 and ultimately reach patients in need.
Many of our trials are funded by federal agencies, such as through the SPORE Grant (zotatifin) from the National Institute of Health (NIH) for our collaborators at UCSF. They are conducted under highest ethical standards in collaboration with our trusted partners, MSKCC, DKFZ, and Baylor College of Medicine.